

Counterfeiters work tirelessly to replicate pharmaceutical packaging components to deliver fraudulent drugs into even the most regulated health systems. Advancements in printed security technology is forever changing, and finding the right solution to protect your patients can be overwhelming.
RLG Healthcare has a team dedicated to staying current in the most efficient and effective anti-counterfeiting options and a documented performance history within several sectors. We can help you protect your healthcare, pharmaceutical, medical device, biotechnology and other healthcare brands with custom-tailored security strategies and solutions. We understand that patient protection is your priority, and we have the capabilities to help you achieve your goals.
RLG Healthcare has designed a system for security and authenticity based on three tiers of protective products and techniques, from simple, low-cost options to smart technologies.
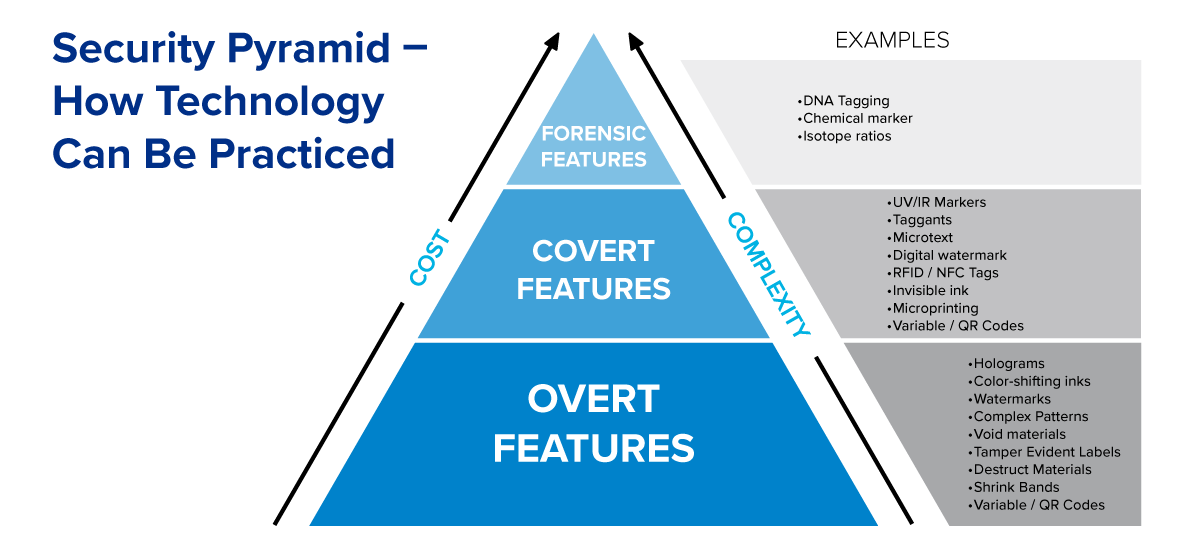
Helping your distributors and consumers identify your products as authentic is becoming increasingly important as the quantity of counterfeit medical products on the market continues to rise. Labeling and packaging solutions can offer you both overt and covert methods for ensuring your products can be identified and traced back to you as the origin.
Including post-printing of variable data
Packaging and labels that make it difficult to open, cartons that have one or more indicators or barriers to entry, and shrink bands that secure a product’s cap or lid – each of these solutions allow you to show signs when a product has been opened or tampered with. These overt packaging solutions help protect your customer and your brand reputation. Our team can help you design the solution that works best for your product and package.

Smart labels provide the most advanced security and protection, with tracking, traceability and anti-counterfeiting features. Smart solutions such as Taggant, NFC or RFID require additional devices or equipment to verify products, while providing a high level of security and protection.
In addition, RFID can assist you with inventory management and distribution, compliance with the US Drug Quality Security Act (DSCSA) requirements for a full interoperable electronic track and trace system and the European Medicines Agency (EMA) recommendations concerning the supply chain tracking.
Taggant based systems for specialized application

Die annually from counterfeit and substandard medication1
Counterfeit medication packages seized in 20212
COVID-19 medical devices units in 20212
Counterfeit medications rose in 2020, up from 10th in 20193
Do you want to protect your consumer base, build loyalty and protect your brand and your bottom line? Click below to learn more about RLG Healthcare and see how we can work with you to safeguard healthcare and pharmaceutical products from tampering, contamination and counterfeiting.
"*" indicates required fields
Copyright © 2024. All rights reserved.